Post Approval Changes and ICH Q12
ISPE Nordic Affiliate invites you to a hybrid event at Novo Nordisk in Bagsværd, Denmark on Wednesday, 29 January 2025 from 08:30 - 17:30.
Location: Novo Nordisk, Smørmosevej 17, 2880 Bagsværd, Building 6BB
Join us for an all-day hybrid seminar on post-approval changes and implementing ICH Q12. Learn how global regulatory frameworks can streamline processes, improve market adaptation, and ensure patient access to critical medicines. Engage in discussions with experts and peers on navigating regulatory challenges and adapting to market needs.
If you attend onsite, enjoy great networking opportunities, explore Novo Nordisk’s impressive facility, and savor a light breakfast, lunch, and networking at Novo Nordisk' Fritidscenter (Laurentsvej 45, 2880 Bagsværd). Plus, have fun with a quiz and competition to keep things lively.
Confirmed Speakers:
- Nanna Aaby Thirstrup – Team Manager, Senior Biological Expert, Danish Medicines Agency (Lægemiddelstyrelsen) – Denmark
- Dr. Markus Goese – Head of EU CMC Regulatory Policy, F. Hoffmann-La Roche Ltd – Switzerland
- Dr. Karim Kacimi – Global Regulatory & Policy, Novo Nordisk – Denmark
- Dr. Andrew Chang – Vice President, Quality and Regulatory Compliance, Global Regulatory Affairs, Novo Nordisk – US
Event Hosts:
- Søren Thuesen Pedersen – Senior Director, Regulatory Policy and Intelligence, Novo Nordisk - Denmark
- Nadia Thorius – Marketing Committee Volunteer, ISPE Nordic Affiliate
Who Should Attend?
This event is perfect for professionals in regulatory affairs, process engineering, quality assurance, quality control, validation, and safety & health. It’s also great for project leaders, managers, students, and emerging leaders, as well as anyone working in pharmaceutical lifecycle management, compliance, or post-approval changes. Whether you want to deepen your expertise or stay ahead in a changing regulatory landscape, this seminar provides valuable insights for all industry levels.
Logistical Details
Important Information for Online Participants: To attend the event online, please make sure to register through NemTilmeld. Once registered, you will receive a link to the event a few days prior to the event date.
Directions for onsite participants:
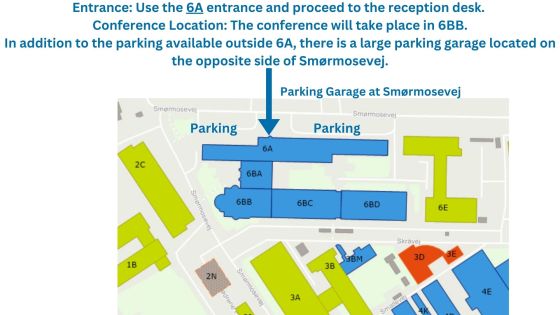
Novo Nordisk, Smørmosevej 17, 2880 Bagsværd, Building 6BB. Use the 6A entrance and proceed to the reception desk. The conference will take place in 6BB.
By car: Drive to Smørmosevej 17, 2880 Bagsværd, Denmark. Limited parking is available outside building 6A, and while there is a parking garage on the opposite side of Smørmosevej, spaces may be limited.
We encourage the use of public transport to ease potential parking difficulties.
By public transportation: S-train: Take the S-train to Bagsværd Station. From there, it is a 12-minute walk to the venue or take Bus 250S: Take bus 250S and get off at the Laurentsvej 45 stop, which is only two stops away. From there, it is just a 5-minute walk to the venue.
Looking for Networking Sponsors:
Important Registration Information
If you need to make any changes to your participation, please update your registration by 21 January 2025 (EOB). This helps us ensure accurate food ordering and minimize waste.
We would like to express our sincere thanks to Novo Nordisk for their generous contribution. If you have registered for the event, please make sure to attend as planned. Your participation is greatly appreciated.
Thank you in advance!
Organizer Contact Information
ISPE Nordic Affiliate
Phone: +45 53 65 91 56
nadiathorius@gmail.com
Information about the Event
Date and time Wednesday 29. January 2025 at 08:30 to 17:30
Registration Deadline Friday 24. January 2025 at 12:00
Location
Novo Nordisk - Auditorium 6B
Smørmosevej 17
Bagsværd
DK-2880
Organizer
ISPE Nordic
Phone +45 53 65 91 56
nadiathorius@gmail.com
 Loading
Loading